BioRestorative Therapies (NASDAQ:BRTX) is a fully integrated regenerative medicine company with preclinical and Phase 2 clinical programs focused on conditioning the bodies’ regenerative potential to treat major diseases more effectively than current standards of care. In addition, BRTX has leveraged its core competencies of cell biology along with the formulation and manufacturing of cell-based products into a unique, proprietary BioCosmeceuticals commercial business within a multi-billion-dollar marketplace.
“We are very well-funded with the resources to catalyze multiple, novel solutions targeting large and growing markets, such as lower back pain, obesity, Type 2 diabetes, and health and beauty,” Lance Alstodt, President, CEO, and Chairman of the Board of BioRestorative Therapies, says in an interview with BioTuesdays.
The global spine pain market was valued at $9.7 billion in 2023 with projected growth at a CAGR of 4.80% from 2023 to 2031. The global weight management market was valued at approximately $142 billion in 2022 with projected growth at a CAGR of 10.0% from 2023 to 2030. The health and beauty market was valued at $378 billion in 2022 with the sector expected to grow at a CAGR of 5.8% between 2023 and 2032.
Mr. Alstodt adds, “While there is no question in my mind that we have great science, demonstrated results, a great team and market opportunities, there is also a clear gap between the value of our future growth prospects and our current market valuation. We are trading at more than a 50% discount to current cash.”
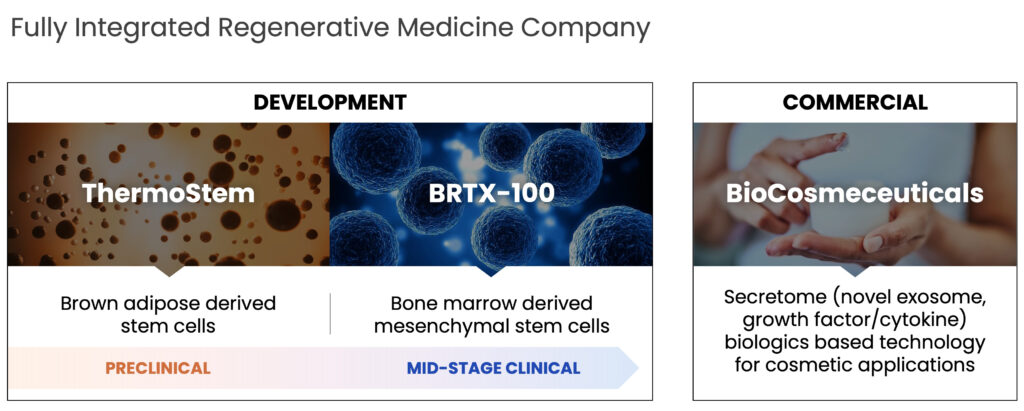
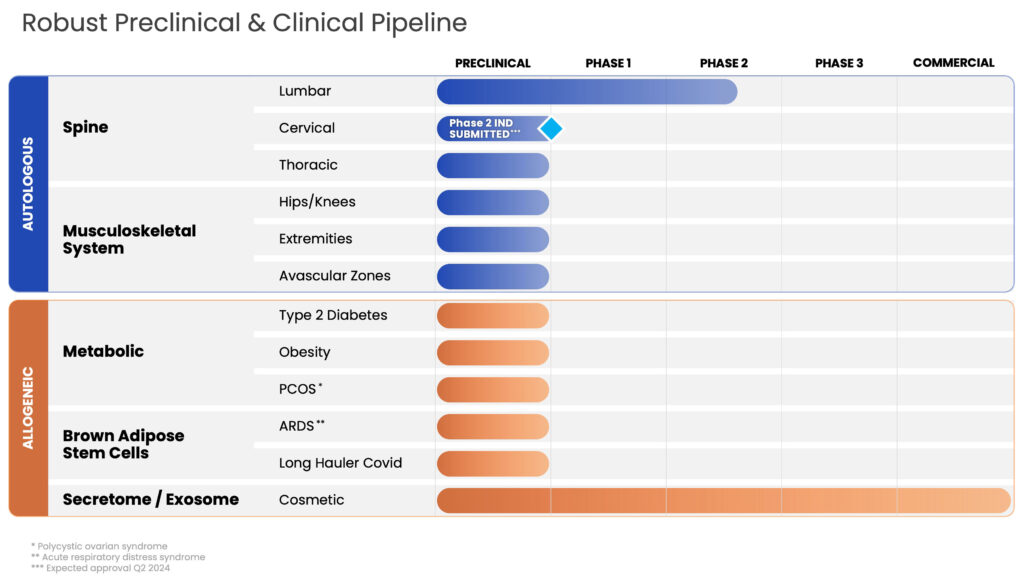
BioRestorative Therapies’ two primary platform technologies include lead cell therapy candidate BRTX-100, a novel, autologous, bone marrow-derived mesenchymal stem cell combined with an autologous platelet lysate which is an injectable product that aims to use the patient’s stem cells to regenerate parts of the musculoskeletal system and ThermoStem, a preclinical program that aims to use allogeneic brown adipose-derived, or brown fat stem cells to address major metabolic diseases such as obesity and Type 2 diabetes.
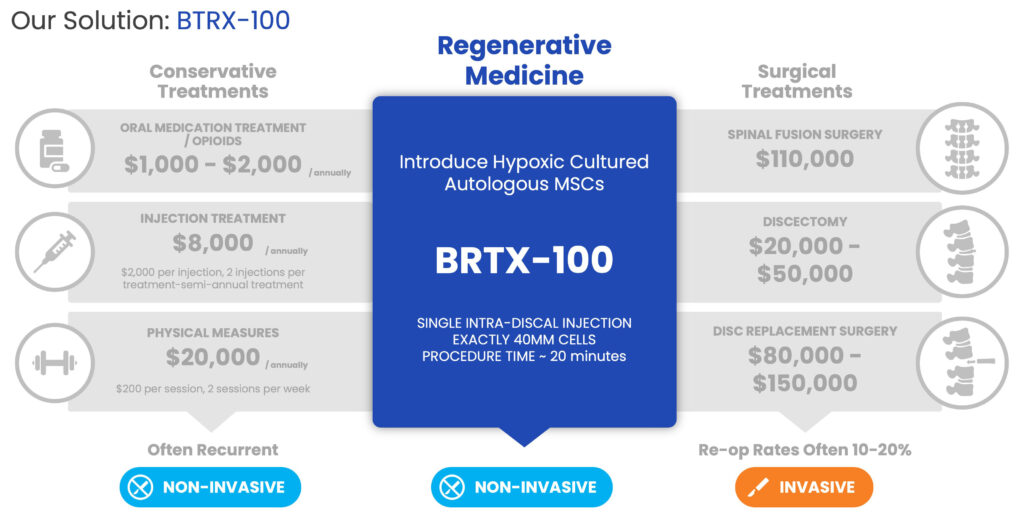
Mr. Alstodt explains, “Our first indication for BRTX-100 is lower back pain caused by degenerative disc disease which is being investigated in an FDA-regulated Phase 2, 99-patient, prospective, randomized, double-blinded and controlled study currently being conducted at up to 15 clinical sites throughout the U.S.” Through a fluoroscopy-guided, minimally-invasive procedure, a total of 40 million stem cells derived from bone marrow are harvested, cultured, and injected directly into the affected disc launching the repair and remodeling process.
“We take the best the body has to offer and supercharge it by culturing and expanding these highly potent cells under hypoxic conditions to mimic and condition them to the harsh, low oxygen environment of the disc in order to maximize cell survivability,” he adds.
Patient-preferred, the technology uses one’s own body to fix the origin of the disc pain with a 100% animal-free, autologous match in a 20-minute, in-office procedure. “BRTX-100 is a safe, cost-efficient regenerative medicine alternative to quite frankly no acceptable alternative solutions, such as conservative treatments or even worse, surgical interventions. This large unmet need can easily be accessed through many providers who have expertise using injectables for disc pain, not only the surgeon community,” Mr. Alstodt says.
With significant domain expertise within the orthopedic and spine-specific sectors, honed through more than two decades of experience in life sciences investment banking, Mr. Alstodt is keenly aware of the opportunity zone for BRTX-100.
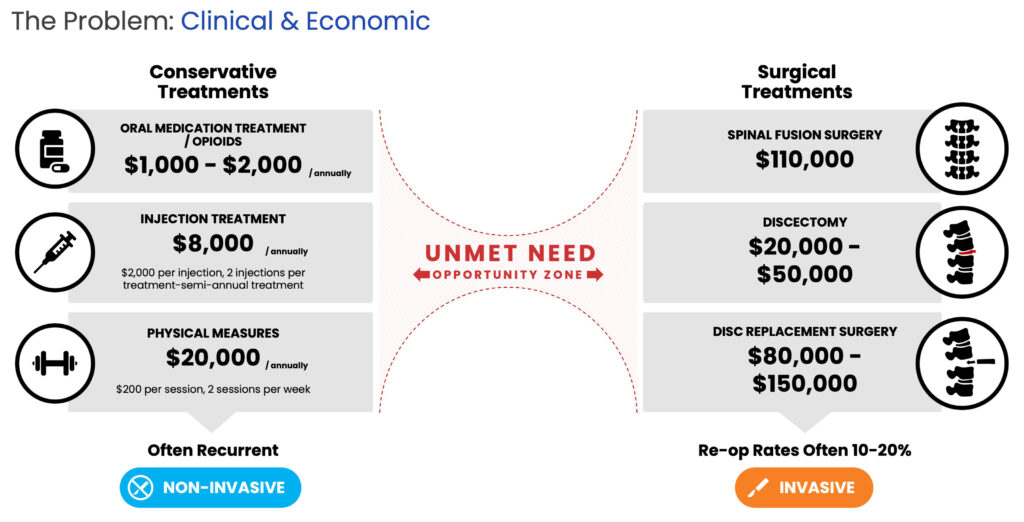
Highlighting that 25 million people suffer from chronic lower back pain in the U.S. alone, Mr. Alstodt informs that half of those people have been diagnosed with disc degeneration. Standard treatment involves either various conservative non-surgical approaches like opioid pain relievers, steroid injections, and physical therapy, all of which ignore the root cause of the problem and have demonstrated limited therapeutic benefits or expensive invasive surgical interventions such as spinal fusion, discectomy, and disc replacement.
“When I was first introduced to BioRestorative Therapies, I quickly recognized BRTX-100’s potential as the holy grail that everyone is chasing to fill the chasm between conservative therapy and surgical interventions with the technology to potentially eliminate lower back pain,” Mr. Alstodt says. “With BRTX-100, we are uniquely positioned to fire on all the cylinders in terms of the technology making sense from a market perspective – cost-efficiency, convenience, patient-preferred, reduced pain, and increased function,” he adds.
Since 2020, the company has seen tremendous growth and change. Formerly an OTC-listed company, Mr. Alstodt successfully organized the company operationally and financially to uplist onto the NASDAQ capital markets enabling access to a larger base of investors and capital resources. Over the last four years, the company has become more communicative with investors as data from its programs has become more available.
For example, BioRestorative Therapies recently presented preliminary BRTX-100 data from its Phase 2 clinical study at the Orthopedic Research Society’s 2024 Annual Meeting. “We have a strong runway for value creation with successful clinical results,” contends Mr. Alstodt. “Although the data presented is still blinded and at an early stage, it is noteworthy that the Visual Analog Scale, Oswestry Disability Index, Roland Morris Disability Questionnaire, and Functional Rating Index collected at 26 and 52 weeks after injection indicated a positive trend compared to the baseline.”
Pain and function questionnaires are used to determine if there have been improvements at weeks 2, 12, 26, 52, and 104 after treatment.
“In addition to safety, improvements as measured by changes in pain and function will play a critical role in the evaluation of BRTX-100 by the FDA for potential Biologics License Application approval,” he adds.
Mr. Alstodt notes that early signals within the BRTX-100 clinical study were very encouraging. “Our primary efficacy endpoints are a minimum of 30% reduction of pain and a minimum of 30% improvement in function. The signals that are inferred from the blinded data could suggest that the endpoints that are being achieved on a small subset of patients are three times greater than the endpoints established with the protocol. These inspirational results with zero notable safety signals are keeping us motivated to enroll as many patients as we can in an expedited manner.”
Looking forward, Mr. Alstodt points out that BioRestorative Therapies has a promising autologous pipeline of activity with indications that can leverage its BRTX-100 technology into additional avascular zones of the body which, similar to the lumbar, are very low in oxygen, circulation, and pH and are traditionally difficult to heal such as knees, hips, shoulders, and other extremities.
“We are literally reaching milestones in the cell therapy space that have never been reached before,” Mr. Alstodt says. “No one in the history of the world has ever been able to successfully insert 40 million mesenchymal cells into a human disc without any dose-limiting toxicities, but we’re doing it. We intend to work with the FDA to seek approval for INDs to initiate studies in some additional areas as we evolve,” he adds.
To address obesity, Type 2 diabetes, and other metabolic conditions BioRestorative’s preclinical program, ThermoStem, uses allogeneic brown adipose-derived stem cells known as brown fat. Elevated levels of brown fat have been shown to increase metabolism and facilitate weight loss. Brown fat may also be responsible for reducing glucose and lipid levels and regulating homeostasis.
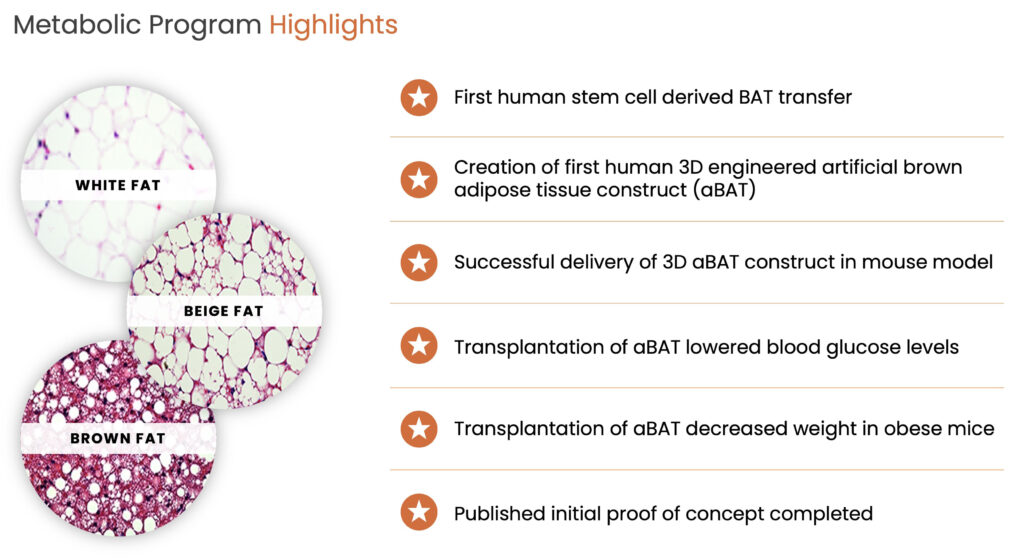
“We are very excited about this program,” Mr. Alstodt says. “It has great proof of concept and we’ve had a variety of meaningful conversations with Big Pharma as we see this product used in connection with pharmacological solutions, such as GLP1s, but taking on a metabolic approach.”
Moreover, BioRestorative was one of the first companies, in collaboration with the University of Utah, to identify and publish data about this novel stem cell population in the peer-reviewed journal, Stem Cell, demonstrating that the use of brown fat cells in small animal models reduced weight, glucose levels, and triglyceride levels in addition to a whole host of therapeutic benefits within the body, he adds.
The company is working with a regulatory advisor to identify opportunities to accelerate this program and maximize the value and power of brown fat cells.
Finally, Mr. Alstodt emphasizes that BioRestorative’s preclinical and clinical programs are expected to be partially funded by the company’s new commercial operation, which leverages its expertise in formulating cell-based products to produce BioCosmeceuticals – a combination of cosmetics and medicines.
BioCosmeceuticals, Mr. Alstodt explains, was born out of a desire to leverage the company’s rich expertise in molecular biology and in-house cell-based product formulation and manufacturing capabilities. “We had already spent $6 million on a cutting-edge, FDA-certified manufacturing facility to control and manage the quality of our therapeutic products,” he says. “So, when we were approached by an esthetics company to formulate a serum that could be used post-treatment to some of their products we engaged in a supplier agreement and began to form a business that we expect will deliver meaningful revenue and profitability, enabling us to become less dependent on the capital markets.”
Overall, Mr. Alstodt says that he believes BioRestorative Therapies is now ready as a company to fully lift the invisibility cloak. “With more than two years of cash on our balance sheet, plus an anticipated healthy revenue stream from our new commercial business, we are in a very strong financial position. And, we will be very forthcoming with news and inflection points coming out of our platform technologies within the next 12 to 18 months.”
• • • • •
To connect with BioRestorative Therapies or any other companies featured on BioTuesdays, send us an email at editor@biotuesdays.com.