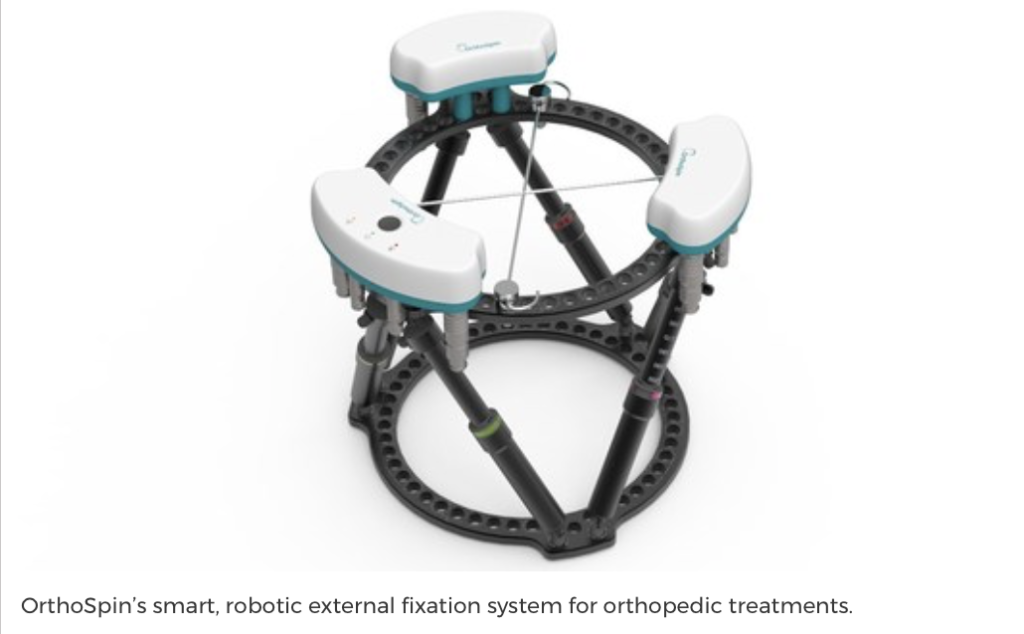
OrthoSpin, a portfolio of company of The Trendlines Group (OTCQX:TRNLY; SGX:42T) received regulatory clearance from the FDA for its smart, robotic external fixation system for orthopedic treatments.
The use of external fixation is a common treatment option for orthopedic-related problems, such as bone lengthening, setting complex fractures, and correcting deformities. The two main challenges associated with these systems are patient compliance and lack of real-time feedback for the physician. In addition, external fixation requires the patient to make multiple manual adjustments every day.
The smart OrthoSpin system automatically makes pre-programmed adjustments, precisely, and continuously, eliminating manual adjustments and patient training. The OrthoSpin external fixation system also delivers a better patient experience and reduces the need for time-consuming follow-ups.
“Our robotic struts eliminate the need for manual adjustment, improve patient compliance and the process is expected to be less painful,” Oren Cohen, CEO, said in a statement.
“OrthoSpin’s system can monitor biomechanical parameters, and in the future will enable the device to automatically send real-time updates to physicians to follow a patient’s progress,” he added. “This allows fine-tuning of the treatment regimen, if necessary, including a personalized treatment plan for each patient.”