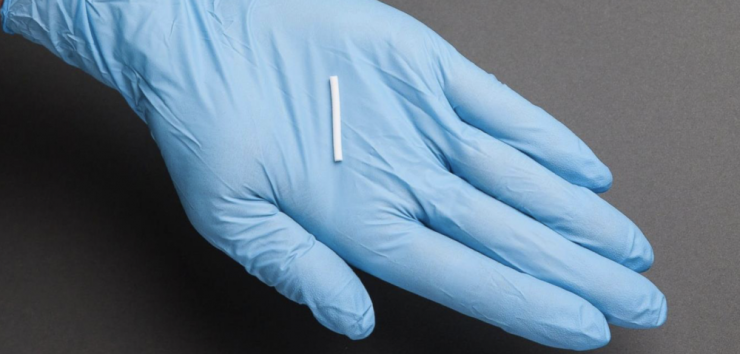
Closely-held Braeburn Pharmaceuticals, which in late May received FDA approval to market the first ever six-month implant of buprenorphine for opioid dependence, is developing a portfolio of long-acting implants and injectables that improve public health and social cost outcomes associated with drug diversion, misuse and non-compliance.
“This is the way of the future,” Behshad Sheldon, president and CEO, says in an interview with BioTuesdays.com. “In 10 years, there will be dozens and dozens of drug products available as long-acting injectables and implantables.”
As a pill-free pharma company aiming to deliver precision medicines in neuroscience, Braeburn has eight programs in three related blockbuster markets: three in opioid addiction, two in pain and two in schizophrenia.
“All of our products are focused around neuropsychiatric indications where team expertise is strong and unmet clinical need is high,” Ms. Sheldon points out.
Braeburn’s opioid maintenance and pain programs are based on buprenorphine, the most commonly prescribed medication for the treatment of opioid dependence. Buprenorphine also has been shown to be effective in treating pain.
For schizophrenia, Braeburn is developing a six-month implant of risperidone, a well-known and trusted product for schizophrenia, and a new chemical entity, ATI-9242.
Seven of the eight programs are proceeding under the FDA’s 505(b)(2) pathway, with potentially lower cost and regulatory risk, she adds. “Our goal is at least one product launch each year through 2020.”
Braeburn’s first approval was Probuphine, a six-month implant for maintenance treatment of opioid dependence in clinically stable patients on 8 mg or less a day of oral buprenorphine. Braeburn licensed Probuphine from Titan Pharmaceuticals (NASDAQ:TTNP) in 2012.
“Probuphine is a new hope for those caught in the fight of their lives in the epidemic that is sweeping America,” Ms. Sheldon contends.
Braeburn’s first approval was Probuphine, a six-month implant for maintenance treatment of opioid dependence in clinically stable patients on 8 mg or less a day of oral buprenorphine.
How it works: four match-stick size implants are inserted under the upper arm and designed to release a steady state of buprenorphine over a period of six months. Along with the freedom of not having to worry about getting medicine every day, Probuphine offers patients addicted to heroin and prescription painkillers continuous, steady dosing, increased compliance and peace of mind that their children are unable to get their hands on the medication.
Probuphine also is expected to tackle the problems of misuse and divergence of buprenorphine, which is the third most confiscated opioid in the U.S. “Stable patients are underserved and in need of innovation with a long-acting buprenorphine implant,” Ms. Sheldon contends.
Braeburn is currently conducting training sessions for healthcare providers and Ms. Sheldon says the response has been overwhelming. There are some 6,000 physicians who prescribe 92% of oral buprenorphine and nearly 5,200 physicians have requested information about Probuphine training.
“We hope to have 2,300 healthcare providers trained by the end of August on proper insertion and removal of the implants and more than 4,000 by the end of 2016,” she adds. The first 10 patients were treated in mid-June.
“I’ve been in this business for 30 years and this is the first time insurance companies have called me to discuss coverage of Probuphine,” Ms. Sheldon points out. Several Blue Cross Blue Shield plans, as well as United Healthcare, were among those that approved reimbursement for the first 10 patients. Cigna, Humana and Aetna also have indicated they will cover the procedure, she adds.
Braeburn plans to use a clinical education field force of 30 people to reach the 5,000-to-6,000 main prescribers of oral buprenorphine.
There are some 2.4 million patients in the U.S. with opioid use disorder to heroin and illicit prescription-opioid use.
There are some 2.4 million patients in the U.S. with opioid use disorder to heroin and illicit prescription-opioid use. Sales of oral buprenorphine were $2-billion in 2014, treating about 30% of diagnosed patients addicted to opioids. The market continues to grow despite four generic competitors.
“We intend to shift the treatment paradigm by replacing daily sublingual products with a suite of complementary long-acting medications for patients at all stages of recovery,” Ms. Sheldon says.
Braeburn also is developing once weekly and once-monthly injections of CAM2038, a FluidCrystal nanotechnology formulation of buprenorphine for the treatment of opioid use disorder to cover all phases of treatment from initiation through maintenance. The company licensed the North American rights to the products from Camurus AB in November 2014, with an option for Asian markets.
According to Ms. Sheldon, the CAM2038 products are designed to ensure proper delivery that minimizes the risks of diversion, abuse, misuse, and accidental exposure.
The CAM-2038 products have been evaluated in three Phase 1/2 clinical trials, which evaluated the safety and tolerability as well as pharmacokinetic and pharmacodynamic properties of the products in a total of 176 individuals, including opioid-dependent patients and healthy volunteers under naltrexone blockage.
In May, Braeburn and Camurus announced positive top line results of a multiple-dose, pivotal Phase 2 study assessing the blockade by CAM2038 of subjective opioid effects of multiple randomized hydromorphone challenges in adults with opioid use disorder.
A key objective of medication-assisted treatment for opioid use disorder is to reduce or eliminate the use of illicit opioids. The results from the Phase 2 study demonstrated that CAM2038 blocks effectively the subjective effects of opioid challenges with hydromorphone, including limiting drug liking.
“This study provided clinical proof-of-concept that CAM2038 will be an effective treatment for opioid use disorder,” Ms. Sheldon contends. “The current opioid crisis demands innovation, and CAM2038’s novel technology is now one step closer to providing physicians and patients with a different approach to treating this deadly chronic disease.” A Phase 3 trial is fully enrolled and will read out in November, she adds.
CAM2038 is a FluidCrystal nanotechnology formulation of buprenorphine for the treatment of opioid use disorder to cover all phases of treatment from initiation through maintenance.
Ms. Sheldon says CAM2038 is also being developed for the treatment of chronic pain. About 50% of oral buprenorphine is used off-label to treat pain. In addition, she points out that more than 100 million people suffer from chronic pain and more than 48 million are treated with opioids.
Long-acting formulations of buprenorphine may benefit patients by limiting peaks and troughs of daily medications; breaking the cycle of pain and minimizing anticipatory worsening of pain; minimizing potential for pill-mills, diversion and abuse; and simplifying drug regimen versus daily medication, she contends.
Braeburn hopes to launch the weekly and monthly versions of CAM2038 as part of its addiction franchise in late 2017 or early 2018, and under its pain franchise in early 2018. It also developing Probuphine as a six-month implant for pain and hopes to launch in late 2018.
In its schizophrenia program, Braeburn is developing a six-month implant of risperidone and a new chemical entity, ATI-9242, which is reverse engineered from clozapine.
“Our product is designed to emulate the best-in-class efficacy profile of clozapine, while significantly limiting its side effects, Ms. Sheldon points out, adding that this novel clozapine analog’s short half-life can be resolved with a long-acting formulation.
According to Ms. Sheldon, brain-imaging studies in animals have shown that ATI-9242 gets into the brain, reaches the correct receptors and at the correct levels.
“We are attempting to replicate those brain-imaging studies in humans and we should know where we stand this month,” she adds. “If all goes well, we will probably begin several Phase 2 trials in the fall to see if we can get a response and we will continue working on a long-acting formulation.”
Long-acting risperidone has successfully penetrated the oral market for schizophrenia, with Risperdal Consta, given every two weeks, and Invega Sustenna as a monthly. “Key opinion leaders, patients and caregivers are receptive to the concept of a six-month implant,” she adds.
Braeburn’s risperidone implant is in a Phase 3 clinical trial, which is expected to read out in 2017, with a possible launch in mid-2018 if it gets fast track approval.