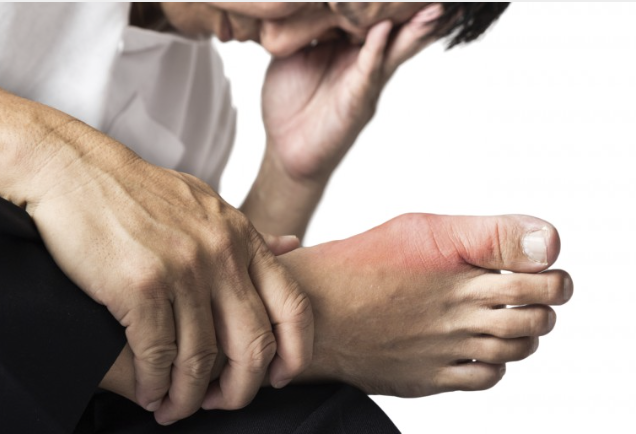
Revive Therapeutics (TSX-V:RVV; OTCQB:RVVTF) hopes to begin a pivotal Phase 2b clinical trial in mid-year with its Bucillamine drug candidate for the treatment of acute gout flares.
“We believe that the Phase 2b data in gout will be a partner-enabling study with a large pharmaceutical company,” CEO, Fabio Chianelli, says in an interview with BioTuesdays.com, noting that the clinical trial should be completed by the end of this year.
Mr. Chianelli explains that Bucillamine is an oral, small molecule drug with a 30-year history as a first-line treatment for rheumatoid arthritis in Japan and Korea. It is sold under the brand name, Rimatil. The drug’s safety has been established in 7,000 subjects from clinical studies and post-marketing evaluations.
“Our strategy, as the name Revive implies, is to repurpose existing drugs that are not approved in the U.S. and Europe and give them new life, with the potential of lower development risk and faster-to-market approval,” he adds.
In addition to gout, Revive also is developing Bucillamine in rare disease programs, including cystinuria, or kidney stones, which is slated to begin a Phase 2 study this year; and Wilson’s disease, which is a buildup of copper in the body.
Mr. Chianelli said the company has partnered with the Bucillamine’s originators for a secured drug supply and exclusive rights to use proprietary non-clinical, human clinical, post-marketing and manufacturing data.
Revive’s Phase 2b study expects to enroll up to 200 patients in the U.S. to assess the efficacy and safety of a low-and-high dose of Bucillamine over seven days, compared with placebo, in the treatment of acute gout flares.
The primary endpoint is treatment responders with a greater or equal to 50% reduction in target joint pain score at 72 hours post-dose, without using a rescue drug. According to Mr. Chianelli, the responder rate from placebo should be 15% to 18%.
In an earlier Phase 2a study with 74 patients, completed in December, Bucillamine was as good as or better than compared with Colcrys (generic name colchicine), a top selling gout drug in the U.S.
Gout is caused by an over production or under excretion of uric acid, leading to the formation of needle-like crystals in joints and soft tissues, and severe pain due to inflammation.
According to IMS Health, sales of colchicine in the U.S. market were approximately $688-million for the 12 months ended August 2014.
In one arm of the study, with a 900 mg dose of Bucillamine, 55% of responders had a ≥ 50% reduction in target joint pain score from baseline at 72 hours post-dose without using a rescue drug, compared with 46% in the comparator arm treated with Colcrys. In addition, Bucillamine demonstrated average pain was reduced by 71% after three days.
Bucillamine was well tolerated and there were no serious adverse events reported in subjects taking Bucillamine in the Phase 2a study.
Mr. Chianelli said Revive has worldwide rights to Bucillamine, with the exception of Japan, Korea and Taiwan. Bucillamine is not approved for the treatment of acute gout flares in the U.S. or Europe.
In January 2016, Revive announced the issuance of a U.S. patent, titled, “The Use of Bucillamine in the Treatment of Gout.” The term of the newly issued patent extends to November 2033.
Gout is caused by an over production or under excretion of uric acid, which leads to the formation of needle-like crystals in joints and soft tissues, and severe pain due to inflammation. Long-term acute gout flares can lead to chronic arthritis and joint damage. Drug options to treat gout include NSAIDS, corticosteroids, as well as Colcrys and Mitigare.
Gout patients also have a high prevalence to other diseases, including hypertension, diabetes, chronic kidney disease and coronary artery disease. Existing gout treatments, however, often have contraindications to these conditions, which can exacerbate complications.
According to Mr. Chianelli, more than eight million adults in the U.S. and 15 million globally have gout, with 40% to 60% of patients failing to reach their uric acid target and suffering from acute gout flares.
In September, Beacon Securities analyst, Doug Cooper, raised his 12-month price target for Revive to $3 from $2. The stock closed at 35 cents on Friday.
According to Mr. Cooper, “one of the key points of Bucillamine is not just its pain reduction attributes, but also that it has benign interaction with other medications. We believe this sets it apart from other gout flare drugs and is very important given the high level of comorbidities associated with gout.”
According to Revive, in a Phase 2a study completed in December, Bucillamine was as good as or better than compared with Colcrys, a top selling gout drug in the United States.
In an earlier report, Mr. Cooper estimated peak sales of Bucillamine for the treatment of gout at about $500-million.
Revive’s lead orphan drug program involves using Bucillamine to treat cystinuria, where stones made from cystine form in the kidney or bladder. Cystinuria, which affects some 20,000 people in the U.S., may result in urinary obstruction, kidney damage, severe infection and potentially lead to chronic kidney disease.
Mr. Chianelli points out that patents have expired on current drug treatments for kidney stones, including Thiola, which is sold by Retrophin (NASDAQ:RTRX) and Valeant Pharmaceuticals’ (NYSE:VRX) Cuprimine. Neither treatment has orphan drug status.
In a 1994 Japanese clinical study, Bucillamine was able to dissolve cystine twice as better than Thiola. Revive received orphan drug status for Bucillamine for the treatment of cystinuria in November, which provides seven years of market exclusivity in the U.S.
Revive’s lead orphan drug program involves using Bucillamine to treat cystinuria, where stones form in the kidney or bladder.
Mr. Chianelli says Revive will be applying for an IND in the second quarter this year to initiate a Phase 2 cystinuria study with up to 20 patients in the third quarter. The study will seek to demonstrate the safety of Bucillamine and a reduction of cystine in the urine over four months. Study results are expected at the end of 2016.
“Bucillamine has the potential to be the first new drug option for this indication since FDA approval of Thiola in the late 1980s,” he adds. The market opportunity for cystinuria is estimated at $500-million in the U.S. alone.
In addition to cystinuria, Revive also is repurposing an anti-depressant drug, Tianeptine, which has been sold in Europe and Asia since the 1980s, in order to treat symptoms of Rett Syndrome.
The rare neurological disorder is characterized by loss of hand motor skills, slowed brain and head growth, problems with walking and breathing, seizures and intellectual disability. Rett affects some 16,000 mostly females in the U.S. and 20,000 in Europe.
According to the Rett Syndrome Research Trust, “imagine the symptoms of autism, cerebral palsy, Parkinson’s, epilepsy and anxiety disorders … all in one little girl.”
Mr. Chianelli says Revive has partnered with Rettsyndrome.org, a leading advocacy group in the U.S., to establish proof-of-concept of Tianeptine. “The first set of results in Rett mouse models demonstrated positive improvement in motor coordination, motion, and clasping/startle.”
Additional preclinical data is expected in the second quarter this year, which will be used to seek orphan drug designation from the FDA in the third quarter and determine its future clinical development program, he adds.
“Overall, our clinical programs, mainly gout and kidney stone treatment, are leading to multiple significant value creating milestones in 2016,” Mr. Chianelli says. “We have a compelling investment proposition, de-risked programs, partnership potential and a low valuation, compared with our peers.”