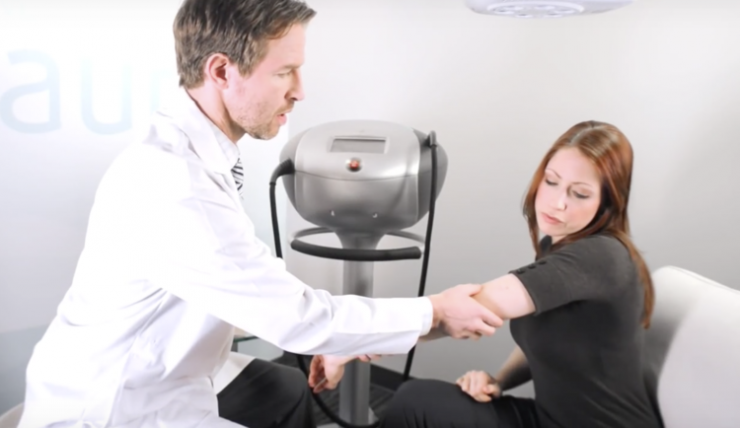
In addition to ramping up distribution of its Aura non-invasive skin cancer detection device this year, Verisante Technology (OTCQX:VRSEF; TSX-V:VRS) is preparing the process to seek FDA approval of Aura, which is already being distributed in Canada and Europe and is approved for sale in Australia.
“We will soon be filing all of our product and clinical data with the FDA in order to seek formal guidance to begin the premarket approval (PMA) process for Aura,” CEO Thomas Braun says in an interview with BioTuesdays.com.
“We are planning on two years to get FDA approval and are prepared to do a clinical study in the U.S., which would either be a pre-PMA study or a postmarket approval study,” he adds. “Either way, we hope to receive clarity from the FDA this year on the number of patients required for the study and the endpoints so we can retain a CRO.”
FDA approval would be a major inflection point for Verisante and its revolutionary Aura device, which recently received a 2013 Bronze Edison Award for innovation. The company also won the prestigious 2013 SPIE Prism Award, dubbed the Oscars of the optics and photonics industry. In 2012, Verisante’s new Core device, which uses the Aura technology along with an endoscopic attachment to aid in the early detection of lung, colon, cervical and other cancers, was voted a Top 10 cancer breakthrough by the Canadian Cancer Society.
The Aura device uses non-invasive Raman spectroscopy to identify spectral changes associated with the biochemistry of skin cancer cells in less than one second. Jointly developed by the BC Cancer Agency and the University of British Columbia, Faculty of Medicine, Aura obtained a statistically significant improvement in the detection of skin cancer in a six-year clinical study of some 1,000 lesions at Vancouver General Hospital, an FDA-certified hospital.
The study, published in Cancer Research, a peer-reviewed journal of the American Association of Cancer Research, showed that in diagnosing skin cancer and pre-cancerous lesions versus benign lesions, Aura achieved sensitivities between 95% and 99%, with specificities between 66% and 24%.
Mr. Braun points out that the study was conducted before Verisante licensed the technology, “so we had no influence on the completely independent study.”
He also indicates that the study results, which were used for Aura’s non-U.S. approvals, are far ahead of the specificity achieved by dermatologists and the endpoints that the FDA required Mela Sciences (NASDAQ:MELA) to demonstrate in order to obtain approval of its MelaFind device for melanoma skin examinations. “So, we think we have a strong case with the FDA.”
Skin cancer is currently diagnosed by visual examination performed by a clinician, followed by a biopsy of suspicious lesions. Previous research has shown that the accuracy of clinicians in correctly diagnosing skin cancer can vary greatly. Biopsy ratios, or the number of non-melanoma lesions that undergo biopsy for each confirmed melanoma, can range as high as 58-to-1, for example.
Mr. Braun contends that Aura will help automate the current diagnostic process through the rapid scanning of skin lesions on at-risk individuals. “Patients will no longer need to suffer through long wait times to see a dermatologist, as scans may be accomplished quickly by trained technicians or assistants,” he says. “Aura will decrease patient wait times, thereby increasing the standard of care, while also decreasing healthcare costs by detecting skin cancer in its early, most easily treatable stages.”
He says Aura further differentiates itself from the competition by detecting a broader range of conditions, including melanoma, squamous cell carcinoma, basal cell carcinoma and actinic keratosis.
Some two million Americans get skin cancer each year, with the cost of treating melanoma alone at $1.5-billion in the U.S. Treating early-stage melanoma costs approximately $1,800, compared with more than $170,000 to treat late-stage disease.
In a statement in January, Dr. Barry Lycka, founder of the Canadian Skin Cancer Foundation, predicted that the device would become an integral part of the practice of any dermatologist who is diagnosing and treating skin cancer. “Clinical study results have shown that the device is fast, accurate and effective in improving patient outcomes.”
Last Friday, Verisante signed its fifth distributor for Aura, Frontiere Medicale Europe LLC, with coverage in the UK and Ireland. Mr. Braun says the company would like to add at least five more distributors for Europe as well as Australia and New Zealand.
“We are at the stage of creating a global distribution network,” he says, adding that the market response has been very favorable. For example, the company has proposals on the table from four potential distributors in Poland.
Following an official launch in April, Clarion Medical Technologies has installed the device in five clinics across Canada and has plans to expand into Quebec and Atlantic Canada.
In addition to Frontiere, Verisante’s European distributors includes: Laserwelt Berlin, covering Germany, Austria and Liechtenstein; bo pharma BV for the Nordic countries of Sweden, Norway, Denmark, Finland and Iceland, in addition to Belgium, the Netherlands and Luxemburg; and Pacifica Handels AG in Switzerland.
Mr. Braun points out that Verisante’s distributors in Germany (where Aura had its official launch in March) and Amsterdam also detail products for Syneron, the largest dermatology equipment maker in the world, and for Canfield Scientific. “We are selling Aura beside premium brands that have a large installed base of clinics.”
The company achieved its initial revenue of $235,000 in the first quarter this year from the sale of Aura devices to its exclusive distributors in Canada and Europe.
With 10 distributors on board later this year, Mr. Braun says the company’s plan is to achieve sales of 10 Aura machines per month during the first year of commercialization and to double sales to 20 per month in the second year. The Aura device sells for $65,000, and the disposable end caps, which are replaced after each use, retail for $10, resulting in a razor and razor-blade revenue model for the company.
Verisante also is using social media via Facebook and YouTube to create awareness of Aura’s benefits. The company’s “Get Screened” video has had more than 40,000 views since May 3, and its Facebook page is currently populated mainly by distributors and clients. Mr. Braun figures that as Aura’s installed base grows, patients will begin posting their experiences on Facebook as well.
To follow Aura into commercial production is Verisante’s endoscopic Core device for the detection of lung cancer. Mr. Braun says the Vancouver General Hospital has collected lung cancer data from 400 patients participating in a clinical study that also is independent of Verisante.
Last November, a pilot study at the hospital demonstrated a remarkable 96% sensitivity and 91% specificity with Core. “This set a new standard for lung cancer detection,” Mr. Braun contends.
He says the company plans to follow the same regulatory pathway as Aura for the Core, gaining approvals in Canada and Europe, and launching the device in non-U.S. markets in 2014.
The company also is in the process of expanding the indications for Core. Mr. Braun says the BC Cancer Agency has received grant funding to start a colon cancer study this year using the Core technology at Vancouver General Hospital. In Asia, a pilot study has been done in Singapore on esophageal cancer, and in China, a study will start this year on nasopharyngeal cancer.
“What this means is that every couple years, we have the potential to launch a new product, and with these new products, we have the potential to be selling 100 units per quarter,” Mr. Braun suggests.